Chemical Reactions
Chemical Reactions
This lesson aligns with NGSS PS3.A
Introduction
Chemical reactions are the processes by which substances interact to form new substances with different properties. A chemical reaction involves the breaking of bonds in reactants and the formation of new bonds in products. This process often results in the transformation of the reactants' physical and chemical properties. This article explores the nature of chemical reactions, their types, mechanisms, and significance in our daily lives.
The Nature of Chemical Reactions
During a reaction, atoms are rearranged, but the total number of each type of atom remains constant, adhering to the law of conservation of mass.
Chemical reactions can be represented using chemical equations, which provide a concise way to express the reactants, products, and the conditions under which the reaction occurs. For example, the reaction between hydrogen and oxygen to form water is represented as:
Types of Chemical Reactions
Chemical reactions can be classified into several categories based on the nature of the reactants and products, as well as the changes that occur during the reaction. The main types include:
1. Synthesis Reactions
In synthesis reactions, two or more simple substances combine to form a more complex compound. These reactions are also known as combination reactions. For example, the synthesis of ammonia from nitrogen and hydrogen is a common industrial process:
2. Decomposition Reactions
Decomposition reactions involve a single compound breaking down into two or more simpler substances. These reactions are often driven by the input of energy, such as heat, light, or electricity. An example of a decomposition reaction is the breakdown of potassium chlorate into potassium chloride and oxygen:

3. Single Displacement Reactions
In single displacement reactions, one element replaces another element in a compound. These reactions typically occur when a more reactive element displaces a less reactive element from its compound. For example, when zinc reacts with hydrochloric acid, zinc replaces hydrogen:
4. Double Displacement Reactions
Double displacement reactions involve the exchange of ions between two compounds to form new compounds. These reactions often occur in aqueous solutions where the ions are free to move and interact. An example is the reaction between silver nitrate and sodium chloride, which forms silver chloride and sodium nitrate:
5. Combustion Reactions
Combustion reactions are characterized by the rapid reaction of a substance with oxygen, releasing energy in the form of heat and light. A common example is the combustion of methane:
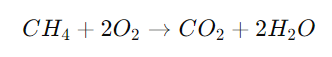
Reaction Mechanisms
The mechanism of a chemical reaction describes the step-by-step sequence of events at the molecular level that leads to the transformation of reactants into products. Understanding reaction mechanisms is crucial for predicting reaction outcomes and designing new chemical processes.
1. Collision Theory
Collision theory states that for a reaction to occur, reactant molecules must collide with sufficient energy and proper orientation. The minimum energy required for a reaction to proceed is known as the activation energy. Not all collisions result in a reaction; only those with enough energy to overcome the activation energy barrier will lead to product formation.
2. Transition State Theory
Transition state theory provides a detailed picture of the molecular changes during a reaction. It posits that reactants pass through a high-energy transition state before forming products. The activation energy corresponds to the energy difference between the reactants and the transition state. This theory helps in understanding the energy profile of a reaction and the factors that influence reaction rates.
3. Catalysts
Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. They achieve this by providing an alternative reaction pathway with a lower activation energy. Enzymes are biological catalysts that play a vital role in accelerating biochemical reactions in living organisms.
Factors Affecting Chemical Reactions
Several factors influence the rate and outcome of chemical reactions, including:
1. Temperature
Increasing the temperature generally increases the reaction rate by providing reactant molecules with more kinetic energy, leading to more frequent and energetic collisions.
2. Concentration
Higher concentrations of reactants typically lead to a higher reaction rate because there are more molecules available to collide and react.
3. Surface Area
For reactions involving solids, increasing the surface area of the reactant (e.g., by grinding it into a powder) exposes more particles to reactants, enhancing the reaction rate.
4. Pressure
For reactions involving gases, increasing the pressure effectively increases the concentration of gas molecules, leading to more frequent collisions and a higher reaction rate.
5. Catalysts
As mentioned earlier, catalysts can significantly increase the reaction rate by lowering the activation energy required for the reaction to proceed.
Conclusion
- A chemical reaction involves the breaking of bonds in reactants and the formation of new bonds in products.
- In synthesis reactions, two or more simple substances combine to form a more complex compound.
- Decomposition reactions involve a single compound breaking down into two or more simpler substances.
- Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process.
Related Worksheets:













